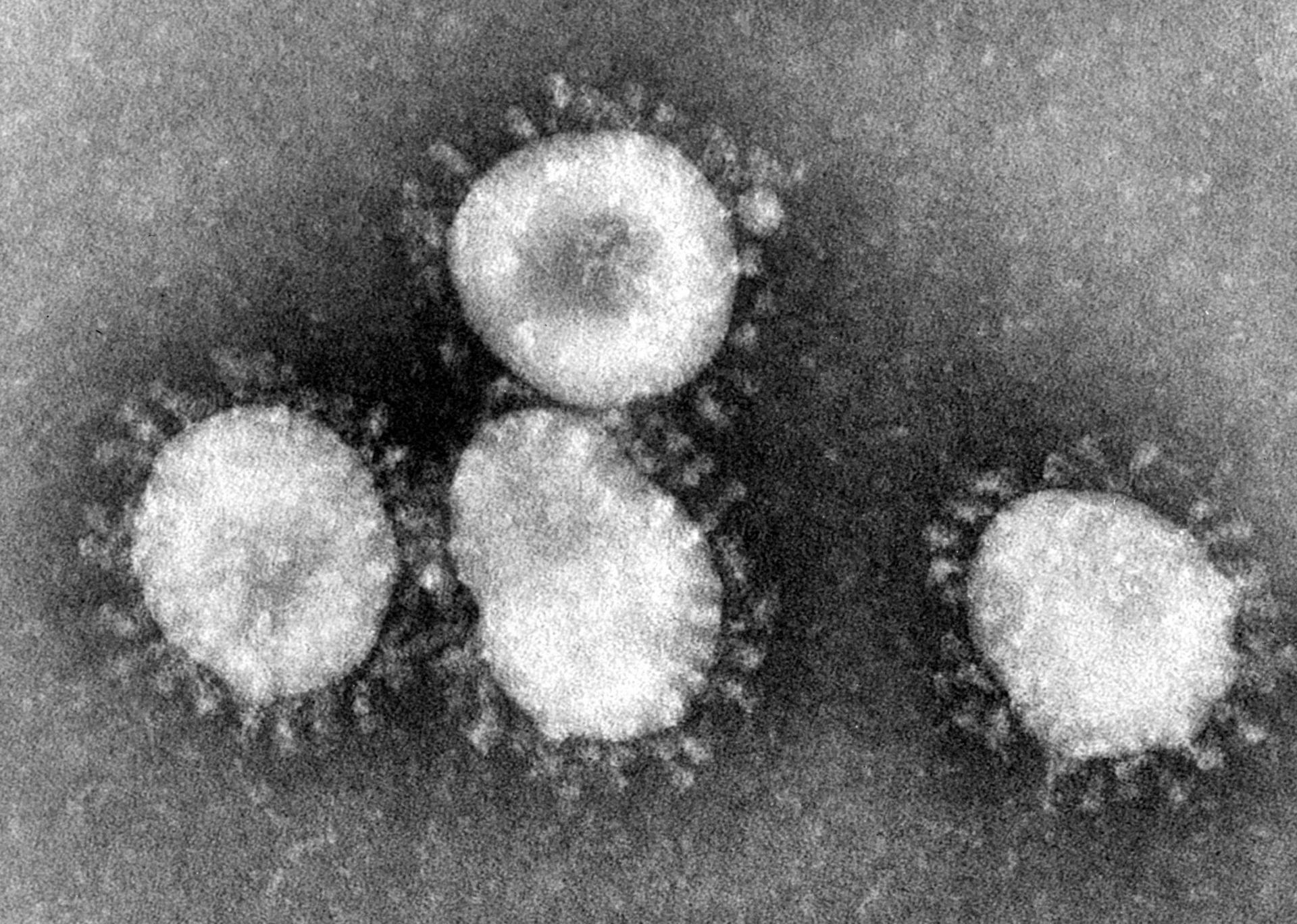
Diagnose COVID-19 & Test for Coronavirus
Coronavirus is spreading at an alarming rate. Here’s what you need to know about diagnosing and testing for COVID-19. Recognizing symptoms early is crucial in preventing the spread and minimizing its effects.
Can COVID-19 be diagnosed?
Yes. The approach to initial management should focus primarily on the early recognition of physical symptoms. These include respiratory and flu-like symptoms, fever, cough, shortness of breath, and breathing difficulties. In addition, environmental and circumstantial factors should also be examined and considered, especially the proximity of the individual to other confirmed cases of COVID-19.
Therefore, at present, the possibility of COVID-19 should be considered primarily in patients with fever and/or lower respiratory tract symptoms who:
-
Reside in or have recently (within the prior 14 days) traveled to areas where community transmission has been largely reported (eg, China, South Korea, Italy, Iran, Japan).
-
Have had recent (within the prior 14 days) close contact with a confirmed or suspected case of COVID-19. Clinicians should also be aware of the possibility of COVID-19 in travelers from or residents in other locations outside of China where community transmission has been reported.
The possibility of COVID-19 should also be considered in patients with severe lower respiratory tract illness when an alternative cause cannot be identified, even if there has been no clear exposure to the virus.
Is there testing available for coronavirus?
The CDC has developed a new laboratory test kit for COVID-19. This test is called the Real-Time Reverse Transcriptase – Polymerase Chain Reaction (rRT-PCR) Diagnostic Panel. The test is made to analyze upper and lower respiratory specimens collected from persons who meet CDC criteria for COVID-19 testing.
CDC’s test kit is intended for use by laboratories designated as qualified (and in the United States, certified under the Clinical Laboratory Improvement Amendment) to perform high complexity tests. The CDC has not yet distributed the testing kit for public administration.
LabFinder is continuously in contact with the existing laboratories in our network. Get breaking updates on COVID-19 testing facilities by subscribing to our live update network
Schedule testing for additional harmful coronavirus strains here: Cold Flu Virus
Where is the best place to get more information?
WHO, CDC, NIOSH, NIH, and FDA websites are the most highly recommended sources for additional information on COVID-19.
Sources:
Center For Disease Control
Science Source
Bored Panda
DebGroup
UpToDate.com


Andy Alem
The LabFinder Editorial Team is behind The Illuminator and The Insider, LabFinder’s consumer and business blogs.
Dr.Robert Segal
Dr. Segal is CEO and co-founder of LabFinder, as well as a board-certified cardiologist. He began practicing medicine in 2002 and has founded several businesses, including Medical Offices of Manhattan and Manhattan Cardiology.